src: i.ytimg.com
- This article is about the physics of atomic lithium. For other properties of lithium, see lithium.
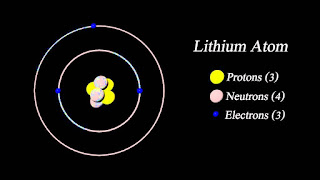
A lithium atom is an atom of the chemical element lithium. Lithium is composed of three electrons bound by the electromagnetic force to a nucleus containing three protons along with either three or four neutrons, depending on the isotope, held together by the strong force. Similarly to the case of the helium atom, a closed-form solution to the Schrödinger equation for the lithium atom has not been found. However, various approximations, such as the Hartree-Fock method, can be used to estimate the ground state energy and wavefunction of the atom. The quantum defect is a value that describes the deviation from hydrogenic energy levels.
Video Lithium atom
Further reading
- W. Zheng et al. / Appl. Math. Comput. 153 (2004) 685-695 "Numerical solutions of the Schrödinger equation for the ground lithium by the finite element method"
Source of the article : Wikipedia